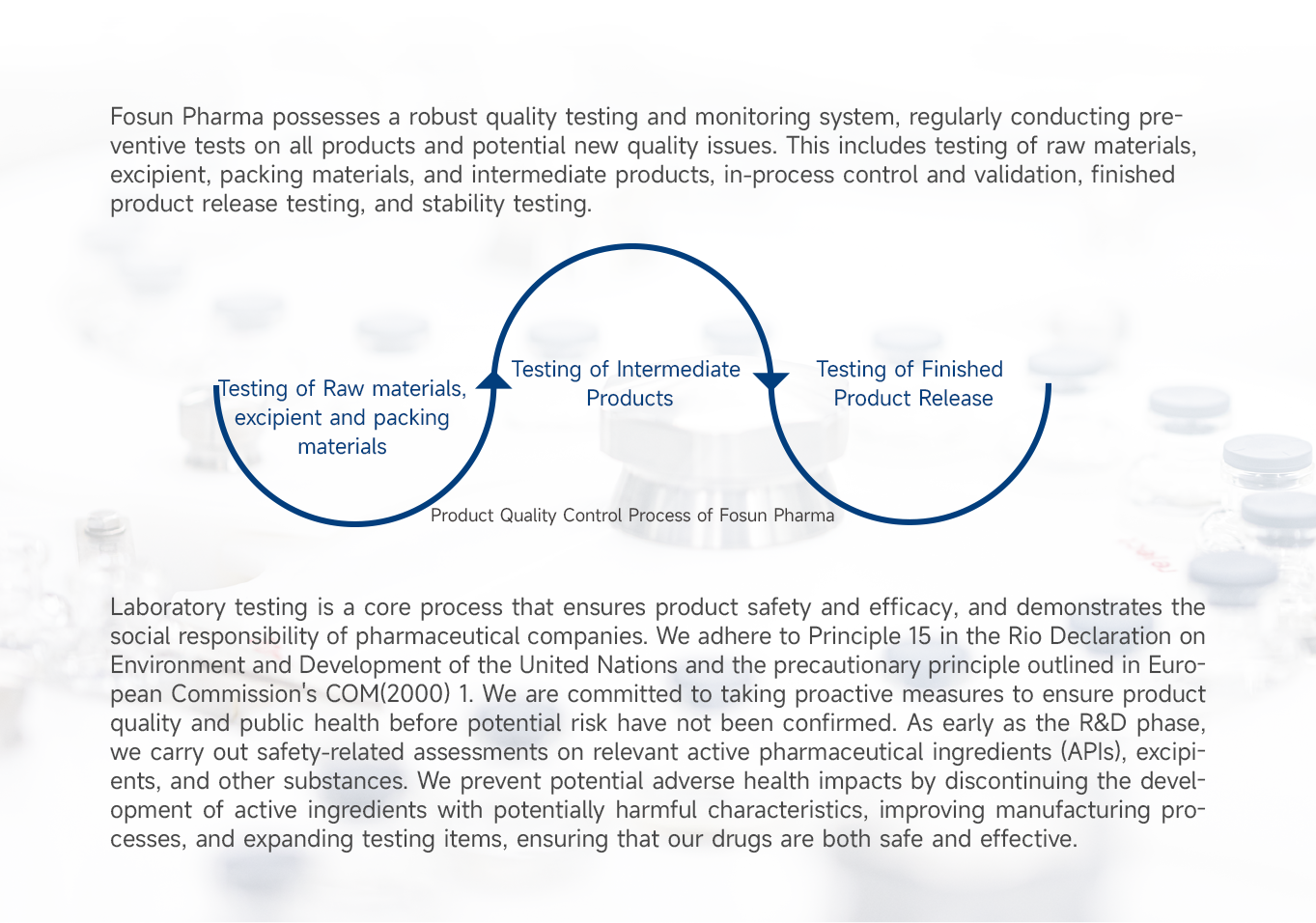
-
Our Company
Shanghai Fosun Pharmaceutical (Group) Co., Ltd. is a global innovation-driven pharmaceutical and healthcare industry group deep-rooted in China

- Fosun Pharma is patient-centered and clinical needs-oriented. The company continuously enriches its innovative product pipeline through independent research and development, cooperative development, license-in, and in-depth incubation. Fosun Pharma improves the research and clinical development capabilities of FIC (First-in-class) and BIC (Best-in-class) new drugs as well as accelerates the R&D and launch of innovative technologies and products.
-
Our Business
Shanghai Fosun Pharmaceutical (Group) Co., Ltd. (Fosun Pharma) directly operates businesses including pharmaceutical manufacturing, medical devices, medical diagnosis, and healthcare services. As a shareholder of Sinopharm Group Co., Ltd., Fosun Pharma expands its areas in the pharmaceutical distribution and retail business.

- Fosun Pharma is patient-centered and clinical needs-oriented. The company enriches its innovative product pipeline through diversified and multi-level cooperation models such as independent research and development, cooperative development, license-in, and in-depth incubation. Fosun Pharma has formed technological platforms for innovative small molecule drugs, antibody drugs, and cell therapy with a focus on key disease areas including oncology and immunomodulation, metabolism and digestive system, and central nervous system. Fosun Pharma also vigorously explores cutting-edge technologies, such as RNA, oncolytic viruses, gene therapy and PROTAC, to enhance its innovation capabilities.
-
R&D
Shanghai Fosun Pharmaceutical (Group) Co., Ltd. is a global innovation-driven pharmaceutical and healthcare industry group deep-rooted in China.

- Guided by innovation and internationalization, the company continuously enriches its innovative product pipeline through diversified and multi-level cooperation models such as independent research and development, cooperative development, license-in, and in-depth incubation. Fosun Pharma improves the research and clinical development capabilities of FIC (First-in-class) and BIC (Best-in-class) new drugs as well as accelerates the R&D and launch of innovative technologies and products.
-
ESG
*data from: Fosun Pharma 2024 ESG and Sustainability Report

- 九游娱乐官网集团将可持续发展纳入公司整体发展战略,重视ESG长效治理能力,建立了由董事会监管、ESG委员会实施、ESG工作小组执行的三级ESG治理架构。董事会ESG委员会负责制定并推进本集团ESG愿景、目标、策略,并向董事会提供建议;ESG工作小组负责梳理拟订重要ESG议题,拟订可持续发展量化目标并跟踪达成进度。ESG委员会和ESG工作小组致力于将ESG理念融入企业运营,提升企业可持续发展能力。
-
Investor Relations
Shanghai Fosun Pharmaceutical (Group) Co., Ltd. is a global innovation-driven pharmaceutical and healthcare industry group deep-rooted in China.

- Founded in 1994, Shanghai Fosun Pharmaceutical (Group) Co., Ltd. ("Fosun Pharma"; stock code: 600196.SH, 02196.HK) is a global innovation-driven pharmaceutical and healthcare industry group deep-rooted in China. Fosun Pharma directly operates businesses including pharmaceutical manufacturing, medical devices, medical diagnosis, and healthcare services. As a shareholder of Sinopharm Group Co., Ltd., Fosun Pharma expands its areas in the pharmaceutical distribution and retail business.
-
News
Shanghai Fosun Pharmaceutical (Group) Co., Ltd. is a global innovation-driven pharmaceutical and healthcare industry group deep-rooted in China.

- Founded in 1994, Shanghai Fosun Pharmaceutical (Group) Co., Ltd. ("Fosun Pharma"; stock code: 600196. SH, 02196. HK) is a global innovation-driven pharmaceutical and healthcare industry group deep-rooted in China. Fosun Pharma directly operates businesses including pharmaceutical manufacturing, medical devices, medical diagnosis, and healthcare services. As a shareholder of Sinopharm Co., Ltd., Fosun Pharma expands its areas in the pharmaceutical distribution and retail business.
-
Careers
Some top talent with strong entrepreneurship gathers here with us.

- We are looking for people that recognize and practice our cultural values, and can learn fast to create continuous value; possess profound professional knowledge of the industry, a wide vision of different regions, and global-level expertise in a certain field. We will also be committed to cultivating international top talent with outstanding performance and great potential.